Oxidation Number of Magnesium
Your body needs both manganese and magnesium to work properly but they perform distinct functions in your body. Journal of Magnesium and Alloys publishes the most papers followed by Materials Science and Engineering A and Materials.

How To Find The Oxidation Number For Magnesium Mg Youtube
Linear relationship is characteristic of metals which form a porous or cracked scale.

. Sodium potassium magnesium with P-B ratio less than 1. Thus everything that leads back to magnesium metal in the previously mentioned. The very few oxidation states of any of the elements are because of the fact that they have very few.
The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction. Using a curved Rh111 crystal here we investigate the effect of A-type square geometry and B-type triangular geometry atomic packing of steps on. Zns 2H aq Zn 2 aq H 2 g Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium.
What changes in this reaction is the oxidation state of these atoms. The oxidation state of carbon increases from 2 to 4. Table of Element Valences.
Studies show oolong tea stimulates fat burning and increases the number of calories your body burns by up to 34. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
Furthermore it is not only determined when an atom loses an electron. A mild and practical Csp3H lactonization protocol has been achieved by merging photocatalysis and magnesium iron nickel catalysis. The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium.
Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change. Heres all you need to know about each essential mineral. Examples of Reduction.
However some of the elements exhibit very few oxidation States. The most accurate reduction definition involves electrons and oxidation number. Now the practical synthesis of a.
While fully ionic bonds are not found in nature many bonds exhibit strong. A diverse range of 2-alkylbenzoic acids with a variety of substitution patterns could be transformed into the corresponding phthalide products. Curved crystals are a simple but powerful approach to bridge the gap between single crystal surfaces and nanoparticle catalysts by allowing a rational assessment of the role of active step sites in gas-surface reactions.
For example common valences of copper are 1 and 2. Based on the mechanistic experimentation and reported prior studies a possible. An important goal for the improvement of certain heterogeneous catalysts is to decrease the amount of platinum required while maintaining high catalytic activity.
Valence is denoted using a positive or negative integer used to represent this binding capacity. As we know the number of electrons in the outermost shell of hydrogen is 1 and in magnesium it is 2. The network visualization among different journals that published at least 10 Mg papers in 2021 is shown in Fig.
On the other hand that of magnesium is 2 as it can lose 2 electrons easily and also attain stability. Oolong tea is high in an amino acid called L-theanine which. Therefore the valency of hydrogen is 1 as it can easily lose 1 electron and become stable.
For example the oxidation of magnesium involves the chemical reaction between magnesium metal and oxygen to form magnesium oxide. The linear law applies to the initial stages of most oxidation before the film is thick enough to be protective and also wherever the protection no longer increases as oxidation continues such as Mo at temperatures where MoO 3 is volatile. The elements that are included in very few oxidation states are zinc and scandium.
In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. This is determined based on the number of electrons that would be added lost or shared if it reacts with other atoms. According to the number of magnesium papers published in 2021 the top 20 journals are listed in Table 1.
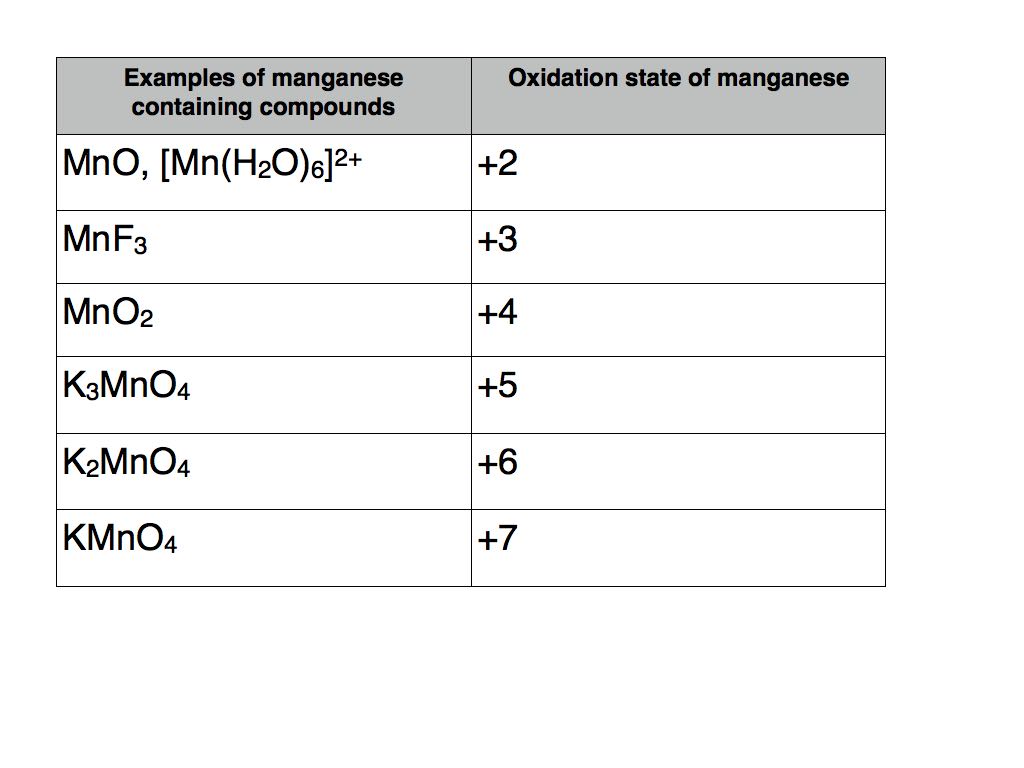
As can be seen in the figure below the total number of electrons in the valence shell of each atom remains constant in this reaction. The word reduction comes from the to lead back sense of the Latin stem. For example magnesium shows a wide range of oxidation states that starts from 2 and goes up to 7 in its various compounds.
Ion electron method or half reaction method.
Oxidation State Examples Online Chemistry Tutor

How To Find The Oxidation Number For Mg In Mgo Magnesium Oxide Youtube

Question Video Calculating Oxidation State Change For Magnesium During Magnesium Combustion Nagwa

No comments for "Oxidation Number of Magnesium"
Post a Comment